Scratching the Surface
Urticaria - also known as hives or wheals, may appear on one part of the body or be spread across large areas[1]. It typically presents with intensely itching, well-circumscribed, raised wheals ranging from several millimetres to several centimetres or larger in size[2]. It happens when histamine and other chemicals are released from under the skin's surface, causing the tissues to swell[1,2]. The intense itch can cause significant impairment in daily functioning and disrupt sleep[2]
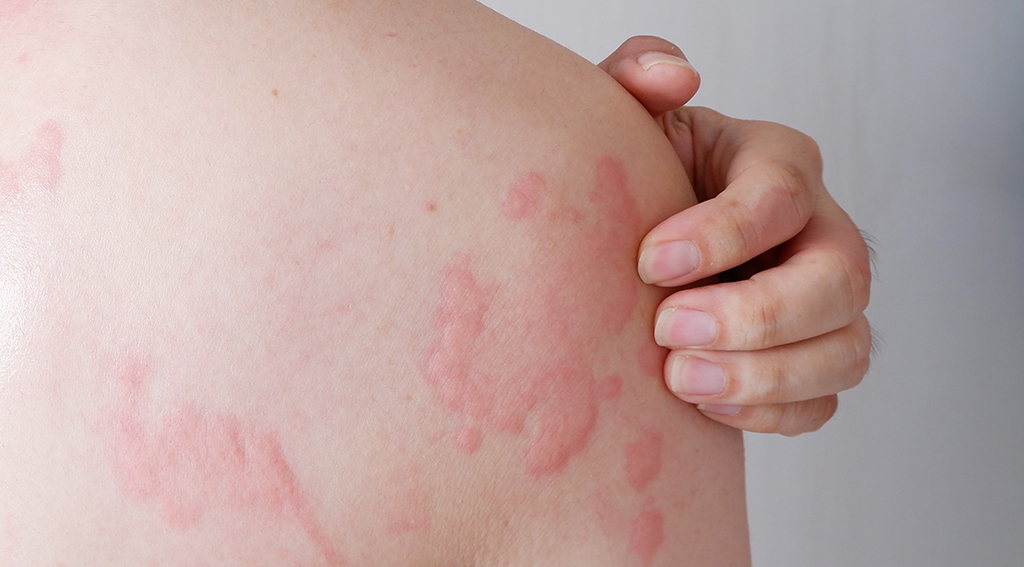
Image Credit: Compassion Dermatology
Treating the root cause
It is essential to investigate the possible triggers of the urticaria so it can be avoided. The common triggers include allergens, food pseudo allergens (foods that contain histamine or salicylates, or cause the release of histamine directly), insect envenomation, medications and infections. Investigations such as skin prick test or blood test might be ordered by the doctor to identify the cause.
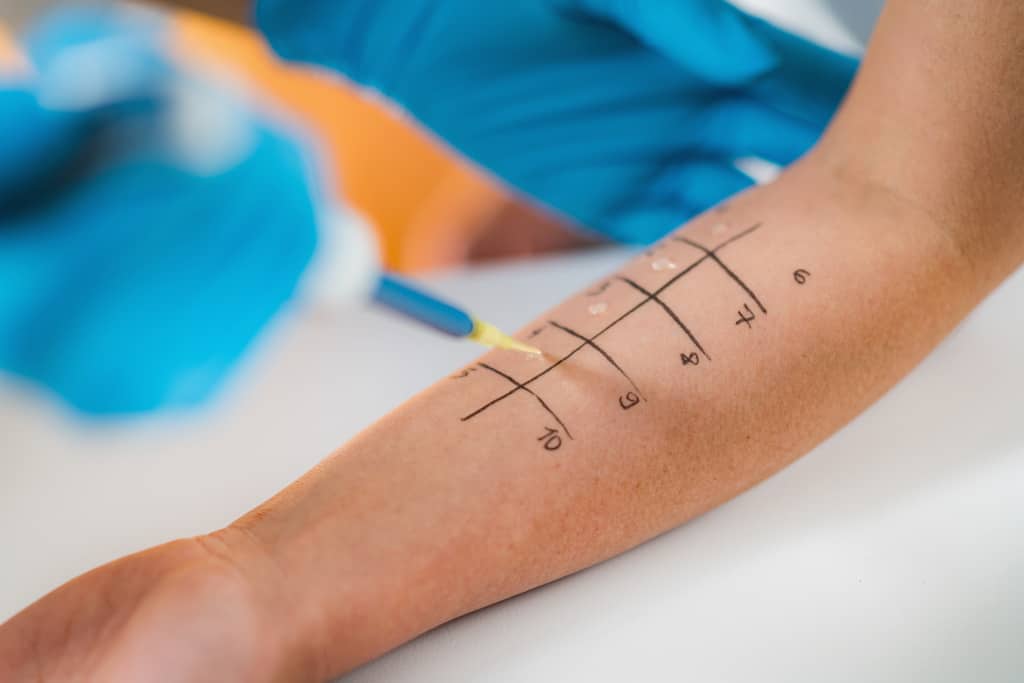
Skin Prick Allergy Testing
Image Credit: International Medical Center
Old is not always gold
Antihistamines (AH) have been available for decades and are recommended by guidelines to treat AR and Urticaria in children. However, there is still a misconception that first-generation AHs are safer than newer, second-generation AHs for use in children owing to physicians’ longer experience of use and currently available safety data[3]. In fact, second-generation AHs have been thoroughly investigated over the years and the results from these studies have shown that second-generation AHs exhibit comparable efficacy to their predecessors, but with lower sedative effects owing to their limited penetration into the brain[4]. In terms of safety, a second-generation AH, has also shown a safety and tolerability profile that was similar to that of a placebo[4,5], allowing its use for prolonged periods without negatively affecting learning and cognitive performance in children.
A tough pill to swallow?
Despite the multiple publications on the efficacy and favourable safety profiles of secondgeneration AHs, [4]many parents may face a common dilemma: young children may dislike taking or are unable to properly swallow medication given in pill form[6,7]. For this reason, medications such as AH have been made available in different and more convenient dosage forms to accommodate the needs of patients in the paediatric group[7]. A variety AHs have been formulated into syrups, whereas orodispersible tablets (ODT) are also becoming more commonly available. Since 2008, the WHO has recommended OT formulations in an effort to increase accessibility of age-appropriate medications for children, particularly in developing countries[7].
Flexibility and convenience
Unlike syrups, ODT formulations are particularly convenient for parents as no measurement is required, allowing for more standardized dosing[6]. Medication in ODT form can also be dissolved in a spoonful of water prior to administration, giving parents added flexibility and convenience[6,7]. Bioequivalence studies available for a second-generation antihistamine in ODT form showed there was no significant difference between the absorption of ODT taken either in oral liquid form or ODT (with or without water)[6,7]. Studies also found that treatment compliance was high in both patients receiving the ODT formulation and placebo, suggesting that an ODT formulation is generally well accepted by children and also convenient for parents[7].
Article source: Menarini [ MY-BIL-202307-023 ]
Reference
- https://www.nhsinform.scot/illnesses-and-conditions/skin-hair-and-nails/urticaria-h ives/
- Schaefer P. Acute and Chronic Urticaria: Evaluation and Treatment. Am Fam Physician. 2017 Jun 1;95(11):717-724. PMID: 28671445.
- Zuberbier, T., Aberer, W., Asero, R., Abdul Latiff, A. H., Baker, D., Ballmer-Weber, B & Maurer, M. (2018). The EAACI/GA-LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy, 73(7), 1393-1414.
- Lynde, C. W., et. al (2020). Multidisciplinary Real-World Experience With Bilastine, a Second Generation Antihistamine. Journal of Drugs in Dermatolocy: JDD, 19(2), 145-154.
- Rodriguez, M., Vozmediano, V., Garcia-Bea, A., Novåk, Z., Yåhez, A., Campo, C., Gamp; Labeaga, L .(2020). Pharmacokinetics and safety of bilastine in children aged 6 to 11 years with allergic rhinoconjunctivitis or chronic urticaria. European Journal of Paediatrics, 1-5.
- Leceta, A., Garcia, A., Sologuren, A., 6amp; Campo, C. (2021). Bilastine 10 and 20 mg in paediatric and adult patients: an updated practical approach to treatment decisions. Drugs in Context, 10.
- Sådaba, B., Azanza, J. R, Garcia-Bea, A., Labeaga, Campo, C., 6amp; Valiente, R. (2020). Bioequivalence evaluation of three pediatric oral formulations Of bilastine in healthy subjects: results from a randomized, open label, crossover study. European Journal Of Drug Metabolism and Pharmacokinetics, 1+5(2), 265-272.









